Evolution and development of the Tetrapod Limb
John Merck
Download cheat-sheet
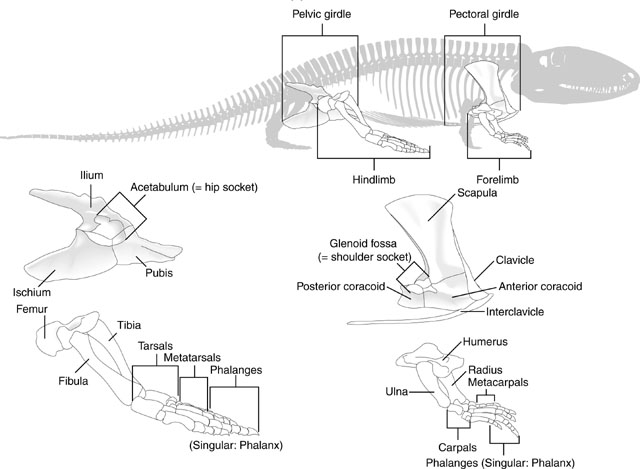
Review: Limb and girdle elements
This should look familiar. Test yourself to make sure you are comfortable identifying elements of the tetrapod forelimb.
But don't forget the hindlimbs:

Eryops sp. right forelimb in anterior view
Forelimb details:
Now some detail:
Manus: the technical term for the "hand."
Tetrapod digits are numbered from I to V (with Roman numerals), with I being the most medial when the manus is in quadruped anatomical position. (E.G.: the human thumb.)
Although there is significant variation over the course of evolution, the tetrapod carpus - the set of carpal elements - ancestrally conforms to a strict pattern where the carpals occupy two distinct rows plus a few:
- Proximal carpals: articulate with the radius and ulna. These are named:
- Radiale: Medial, articulating with the radius.
- Ulnare: Lateral, articulating with the ulna.
- Intermedium: Between ulnare and radius.
- Centrale 4: When present, articulates with intermedium.
- Distal carpals: articulate with the metacarpals for each digit. These are numbered (with arabic numerals) to correspond to the digit with which they are associated.
- Centralia: (Singular "centrale") Ancestrally, tetrapods had most of a third row between the distal and proximal carpals. These are numbered from medial to lateral. (In tetrapod evolution, these are often reduced or lost.)

Eryops sp. right hindlimb in anterior view
Hindlimb details:
Pes: the technical term for the "foot."
The ancestral pattern of the tetrapod tarsus is similar:
- Proximal tarsals: articulate with the tibia and fibula. These are named:
- Tibiale: Medial, articulating with the tibia.
- Fibulare: Lateral, articulating with the fibula.
- Intermedium: Between fibulare and tibia.
- Note: In amniotes, the tibiale, intermedium, and at lease one centrale fuse to form the astragalus, but that is a story for later.
- Distal tarsals: articulate with the metatarsals for each digit. Numbered as above.
- Centralia: Up to four, numbered from medial to lateral as in the manus.

Dimetrodon sp. forelimb (above) and hindlimb (below)
Limb regions:
Because the overall patterns of the fore and hindlimbs are so similar ancestrally, it is convenient to refer to their general regions collectively. Thus, each fore and hindlimb has a:
- Girdle: pectoral or pelvic
- Stylopodium: the upper limb, with a single bony element, the humerus or femur.
- Zeugopodium: the lower limb, with two bony elements, the radius and ulna or tibia and fibula.
- Autopodium: the manus or pes.

Eusthenopteron foordi forelimb
Development and Evolution
How did this clear and highly conserved pattern evolve? Now we have a problem. In the skeleton of the pectoral fin of Eusthenopteron foordi, an aquatic non-tetrapod we see homologs to some of the elements of the tetrapod forelimb. (See if you can identify.) However the different regions are not strongly differentiated. It gets even worse when we look at more primitive sarcopterygians:
Development and Evolution
Of course, this in no way resembles the forelimb skeleton of actinopterygians. The most we can say is that sarcopterygian pectoral fins seem to have:- a metapterygial axis: a row of primary median elements.
- preaxial fin rays: anterior to them.
- postaxial fin rays: posterior to them.
The identification of homologs to the non-tetrapod metapterygial axis has been a research priority for over a century, but the breakthrough came with Shubin and Alberch, 1986. They noted that:
- Formation of cartilages proceeded from proximal to distal in the limb bud.
- At the distal end of a chondrification in a limb that was continuing to develop, two things could happen:
- Segmentation: a single new cartilage could arise
- Bifurcation: two new cartilages could arise
- However, in the stylopodium and zeugopodium of tetrapods, only the postaxial elements induced bifurcations. This is similar to the fin skeleton of Eusthenopteron.
- In the autopodium, the pattern reversed, with preaxial elements inducing bifurcation. Postaxial elements then segmented into elements of the digits. The bifurcating preaxial elements make up the digital arch.
One odd thing: Shubin and Alberch's scheme didn't seem to accommodate digit V, which seems to form separately. How does it relate to the digital arch?

The limb bud
- Mice
- Chickens
- Skates
- Zebra-fish
All gnathostomes surveyed organize their developing limb buds similarly:
- Growth is from proximal to distal
- Distally, differentiation of skeletal elements occurs in an apical ectodermal ridge
- A Zone of Polarizing Activity (ZPA) at the posterior end of the apical ectodermal ridge coordinates the differentiation of digits.
The Zone of Polarizing Activity: Reviewed by Shubin, 2008. (Discuss and review.)
Limb segment identities: Hox genes 9 - 13 are expressed in tetrapod limbs. Remember:
- Hox genes occur in four paralogous clusters termed Hoxa, Hoxb, Hoxc, and Hoxd. ("Paralogous" = sharing a common ancestry through a gene duplication event. Whereas homologous things (structures, genes, etc.) are related through lineage splitting events.)
- Paralogous genes in each cluster are numbered.
- Hoxa9 is expressed in the girdles
- Hoxa10 in the stylopodium
- Hoxa11 in the zeugopodium
- Hoxa13 in the autopodium
What about Hoxd? In aquatic choanates, each non-terminal segment of the limb axis is followed by two elements, the next axial segment, and a preaxial (in front of the axis) radial branch. The result is:
- A primary axis (aqua)
- A preaxial field (white)
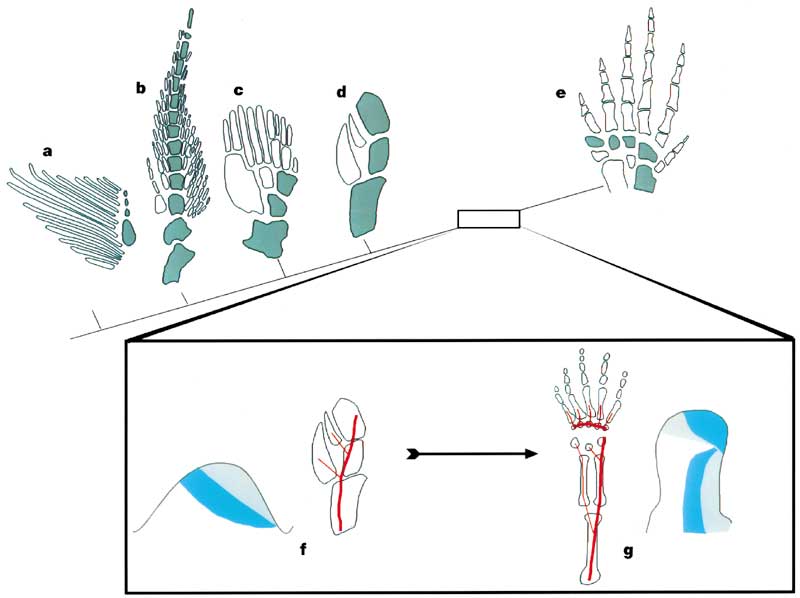
- Cladoselache (a primitive chondrichthyan)
- Neoceratodus (the living Australian lungfish)
- Sauripteris (a primitive sarcopterygian)
- Panderichthys (a choanate)
- Tulerpeton (a stegocephalian)
Schematics F and G show the expression of the HOX genes in the embryonic limb buds of zebra-fish (with no autopodium) and land vertebrates (with an autopodium) respectively. Significantly the domains of the Hoxd11 and Hoxd13 genes are reversed at the end of the land vertebrate limb - corresponding to the shift from pre- to postaxial bifurcations. This switch in Hox gene domain apparently defines the developmental identity of the autopodium.
The result:
- long slender proximal elements (E.G.: humerus, radius, ulna)
- long slender distal elements (E.G.: metacarpals, phalanges)
- small blocky elements in between (E.G.: carpals)
This pattern is congruent with the zones of activity of Hoxa. In non-tetrapods, the domain of Hoxa11 overlaps that of Hoxa13 all the way to the end of the fin. In tetrapods, the overlap is reduced to the zone in which carpals and tarsals form.
As some non-tetrapods show, the coordination of Hox domains and promotors may not have evolved lockstep.
Step three - stopping the formation of digits:

Hindlimb of Ichthyostega
- primes the ectodermal apical ridge to produce digits
- promotes the expression of bone morphogenic protein 8 (Coded on Hoxb8).
Final enigmas:
What happened to the fin rays? Good question. Fin rays arise from neural crest ectoderm that condenses in the apical fin fold of the embryonic limb bud in creatures that have them. So far, all fossil stegocephalians have either fin rays or digits on their paired limbs, but never both. Nakamura et al. 2016 have show that fin rays and digits arise from the same precursors, but that the development of fin rays requires the actions of an autodial Hox13 enhancer.
What about the space between the fingers? The presence of bony digits doesn't guarantee the presence of separate fleshy fingers and toes. To make these requires apoptosis - programmed cell death - of the cells between the digits in the growing limb bud. Where in tetrapod evolution that ability arose is unclear.
Law of similarity? Generally speaking the fore and hindlimbs of tetrapods are similar because their growth is governed by the same developmental mechanisms. Nevertheless, in both evolution and ontogeny, the forelimbs seem to lead the hindlimbs somewhat. Might we eventually encounter a fossil sarcopterygian with digits on its forelimbs and fin rays on its hindlimbs?
What about digit V? Recall that digit V does not seem to be part of the digital arch. Genomics support this inasmuch as experiments in which Hoxa13 and Hoxd13 are made inoperative result in the absence or malformation of the digital arch, but do not prevent that formation of digit V. Carroll 2009 notes the presence of isolated postaxial fin rays in non-tetrapods like Eusthenopteron (above) and Tiktaalik. Could one of these be homologous to digit V? Why would it look like a digit? Maybe because it was near the zone of expression of the protein Fgf8 (fibroblast growth factor 8) that induces segmentation of digits to produce a sequence of phalanges.
- Robert Carroll, 2009. The Rise of Amphibians: 365 Million Years of Evolution. Johns Hopkins University Press, Baltimore. 360 pp.
- Jennifer Clack, 2014. Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press, Bloomington. 523 pp.
- Marcus Davis, Randall Dahn, Neil Shubin, 2007. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature 447, 473-476.
- Tetsuya Nakamura, Andrew R. Gehrke, Justin Lemberg, Julie Szymaszek, and Neil H. Shubin, 2016. Digits and fin rays share common developmental histories. Nature 537, 225-228.
- Neil Shubin, 2008. Your Inner Fish: A Journey into the 2.5-Billion Year History of the Human Body.Vintage Books, USA. 237 pp.
- Neil Shubin, 2008. Your Inner Fish: A Journey into the 2.5-Billion Year History of the Human Body.Vintage Books, USA. 237 pp.
- Neil Shubin and Pere Alberch, 1986. A morphogenetic approach to the origin and basic organization of the tetrapod limb. Chapter 5 in Evolutionary biology Springer, USA. pp. 319-387
- Joost M. Woltering, Daan Noordermeer, Marion Leleu, Denis Duboule, 2014. Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. PLOS Biology, January 21, 2014DOI: 10.1371/journal.pbio.1001773.
- Jennifer Clack, 2014. Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press, Bloomington. 523 pp.