Minerals are linked in many important ways with the global ecosystem. Minerals are the main sources of elements needed for the development of civilization and elements that are pollutants or essential plant nutrients. These elements are released from minerals through chemical weathering and anthropogenic activities, such as mining and energy production. Minerals also play key roles in the biogeochemical cycling of the elements, sequestering elements and releasing them as the primary minerals in crustal rocks undergo various structural and compositional transformations in response to physical, chemical, and biological processes that produce secondary minerals and soils. The mineral-water interfaces are the locations of most chemical reactions (e.g., adsorption/desorption, oxidation/reduction and precipitation/dissolution) that control the composition of the natural environment, including the composition of natural waters. To improve the fundamental understanding of these processes, our group uses modern molecular-scale analytical and theoretical methods to acquire molecular-scale information about minerals, including their bulk structures and properties, formation and transformation. The minerals of our primary interests are Mn and Fe oxides that are highly reactive and actively participate in complex redox and mineral-water interfacial reactions. These reactions strongly affect biogeochemical cycles and pollutant dynamics in Earth's critical zone.
Our current focus is on Mn oxides that are common in the environment, imposing significant impacts on many critical biogeochemical processes owing to their extraordinary sorption and oxidation properties. We try to address what and how geochemial processes affect Mn oxide structure, formation and transformation. Some of our recent work are introduced below.
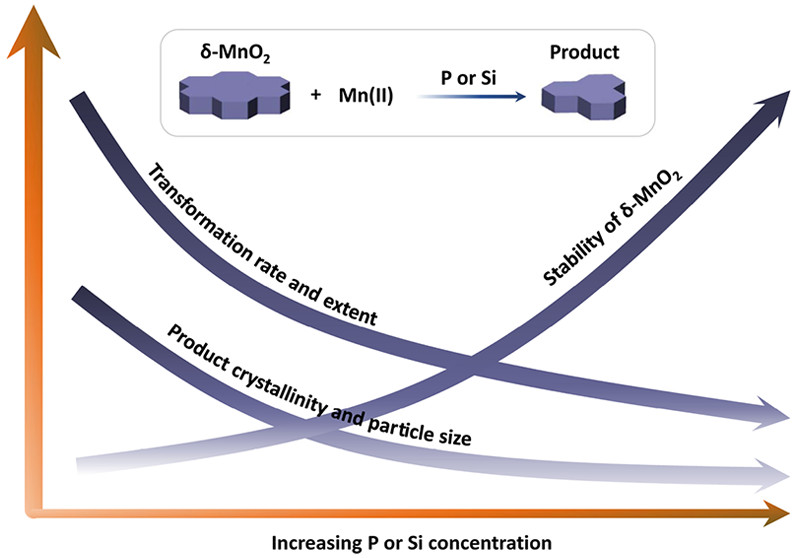
1) Inhibition of oxyanions on redox-driven transformation of layered manganese oxides (Yang et al., Environ. Sci. Technol., 2021)
2) Metal Adsorption Controls Stability of Layered Manganese Oxides (Yang et al., Environ. Sci. Technol., 2019)
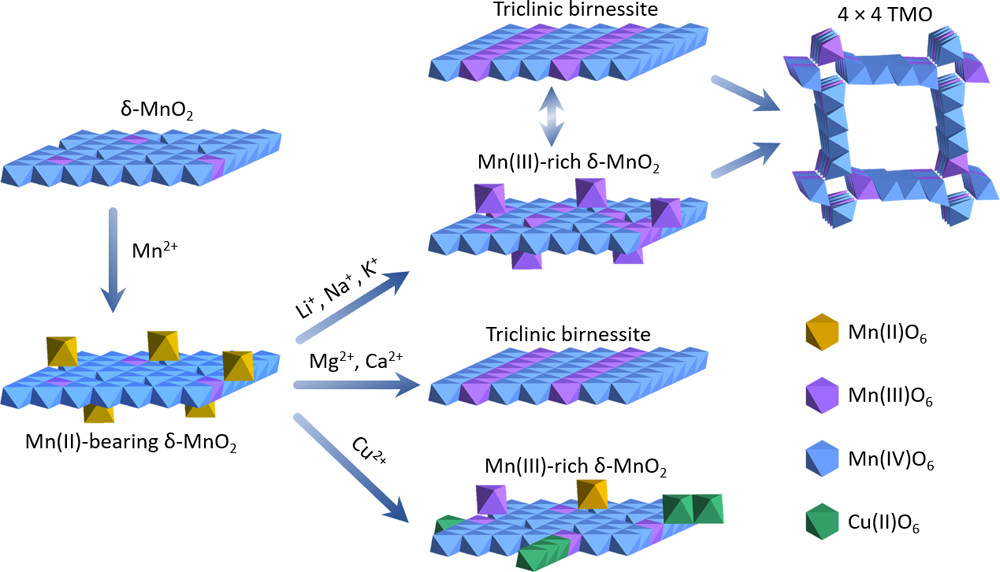
Transformation pathways of Mn(II)-bearing δ-MnO2 to other phases in different systems.
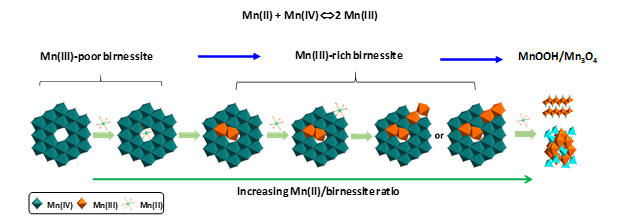
3) Trivalent manganese on vacancies triggers rapid transformation of layered to tunneled manganese oxides (TMOs): Implications for occurrence of TMOs in low-temperature environment (Yang et al., Geochim. Cosmochim Acta., 2018)
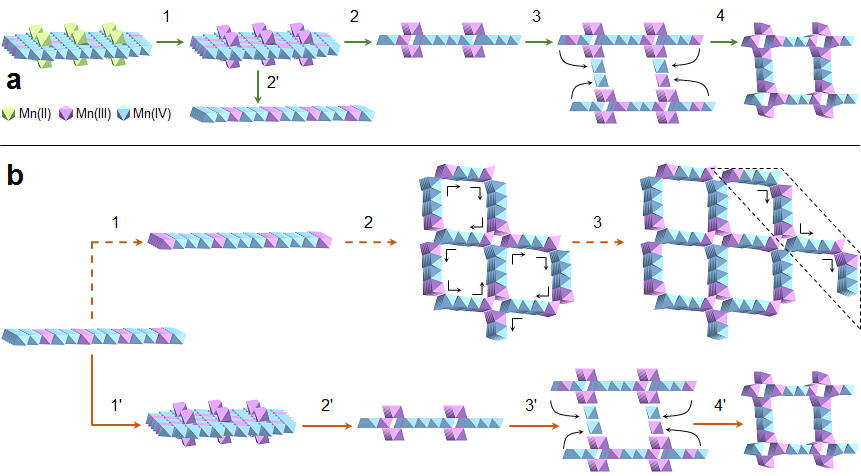
4) Effects of metal cations on coupled birnessite structural transformation and natural organic matter adsorption and oxidation (Wang et al. Geochim. Cosmochim. Acta., 2019)