Key Points:
•The record of eukaryotes of increasing complexity starts ~ 1.6 ga.
•Eukaryotic cells are composites of prokaryote-grade organisms that have colonized one another by endosymbiosis.
•Eukaryote phylogeny is unsettled, but the groups Archaeplastida, Opisthokonta, and Chromalveolata are well-supported.
•Major fossil-forming groups include: Rhodophyta (red algae), Viridiplantae (green plants), Radiolaria, Foraminiferida (foraminiferans), Alveolata (incl. dinoflagellates), Bacillariophyceae (diatoms), Coccolithophyceae (coccolithophorids), choanoflagellates, and Metazoa (animals).
•Each group listed above yields distinct fossils and has its own life-history/reproductive strategy.
•Choanoflagellates appear to be the closest relatives of metazoans.
"The view of evolution as a chronic bloody competition among individuals and species, a popular distortion of Darwin's notion of "survival of the fittest," dissolves before a new view of continual cooperation, strong interaction, and mutual dependence among life forms. Life did not take over the globe by combat, but by networkiing. Life forms multiplied and complexified by co-opting others, not just by killing them."
(Lynn Margulis, 1986. Microcosmos: Four Billion Years of Evolution from Our Microbial Ancestors)
Some groups of eukaryotes actually lack some typical organelles, but these seem to be secondary losses:
- Pelobionta: Lacking mitochondria, flagella, and other typical eukaryotic organelles; and able to tolerate a low[O2]. Trumpeted during the late 20th century as representing an early stage of endosymbiosis, but now considered close to amoebas and thought to have lost these organelles secondarily.
- Diplomonada (including Giardia) possess organelles that seem to represent degenerate mitochondria that have lost their respiratory function (Van der Giezen and Tovar, 2005.)
Recall that such Eukaryotic abilities as:
- The use of oxygen as an electron receptor in cellular respiration.
- Sexual reproduction through alternation of diploid and haploid life stages
May have been adaptations to the rise of aerobic environments during the Great Oxygenation Event. Another trend we see among eukaryotes is increasing versatility in the management of protein synthesis.

An operon with: 1.) RNA polymerase, 2.) Repressor protein, 3.)Promoter, 4.) controlling region.
Operons: We mentioned how information in DNA is transcribed into proteins previously. We didn't discuss what triggers the process. The magic of protein synthesis is that DNA is only transcribed into mRNA when the protein it codes for is needed. E.G.: The bacterium Escherichia coli freely metabolizes glucose, but if glucose is lacking, and lactose is present, it can metabolize lactose by producing an enzyme, beta-galactosidase, that breaks lactose down in to glucose and galactose. But how does it know when to make beta-galactosidase?
The work of François Jacob and Jacques Monod established the answer (Nobel Prize in 1965):
- Adjacent to the gene for beta-galactosidase (and other proteins involved in lactose metabolism) is a small controlling region gene to which a protein, the lac repressor binds like a padlock.
- Upstream from it is another controlling region, the promoter to which RNA polymerase binds in order to begin transcribing the DNA.
- Even if RNA polymerase binds to the promoter, the lac repressor blocks it from unzipping the DNA and making mRNA.
- But the magic: Lactose, itself, binds to the lac repressor causing it to fall off of the DNA strand and allowing RNA polymerase to do its thing.
- Once all the lactose has been metabolized, the lac repressor reattaches and transcription ceases.
The operon: The stretch of contiguous DNA containing the promoter, the controlling region, and the genes coding for beta-galactosidase and other proteins involved in lactose metabolism.
Animation.
Big Point: Somewhere on the genome, the lac repressor protein is, itself, coded for in another operon by a repressor gene whose activity may be regulated by yet other other repressors:
- In prokaryotes, this system of operons is relatively simple
- Among eukaryotes, it is complex, with many layers of regulatory and structural genes and their protein products.
Eukaryotes, thus, display a degree of metabolic plasticity that prokaryotes lack.
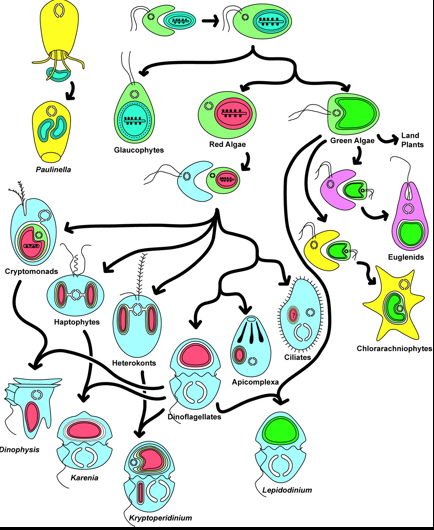
Eukaryote Phylogeny
The large-scale phylogenetic pattern of Eukaryota is contentious. A consensus phylogeny of recent hypotheses appears at right. Within it are named groups whose monophyly is well established.
Note: The phylogram to which this cladogram links superimposes the phylogeny of fossil-forming groups on to the stratigraphic record. Typically we could infer minimum divergence ages with some confidence. In the case of Eukaryotes, however, this is perilous as so few yield fossils. El Albani et al. 2014, for instance describe "colonial" organisms with features associated with colonies of eukaryotic cells in the 2.1 Ga Francevillan B Formation of Gabon. These colonies were not organized into bodies in the manner of multicellular fungi, plants, and animals, but were akin to congregations of independent cells like slime molds.
Hypotheses of the phylogeny of eukaryotes are subject to frequent revision, so we focus on descriptions of major fossil-forming groups, rather than the broad patterns. And yet a few words on large groups:
- Archaeplastida: Includes Rhodophyta (red algae) and Viridiplantae (green algae and plants). Potential synapomorphies:
- Chlorophyll a
- Presence of cellulose cell walls
Interesting plesiomorphy: Organelles including chloroplasts have only two cell membranes, indicating that they result from a single episode of endosymbiosis.
- Opisthokonta: Includes Fungi and Metazoa (animals). Potential synapomorphy:
- Collagen as structural protein
- When a flagellum is present it is in a posterior position and pushes the cell. (E.G. sperm cell.)
- Alveolata: Includes Dinoflagellata. Potential synapomorphy:
- Outer cytoplasm packed with alveoli (sacs) that can form a membrane or armor plates.
- Ochrophyta: Includes Phaeophyceae (brown algae) and Basillariophyceae (diatoms). Synapomorphies are molecular.
- Rhizaria. Includes Foraminiferida and Radiolaria. Potential synapomorphies:
- Slender pseudopodia
- Morphology of mitochondria
Major microfossil-forming groups:
Odd-balls:
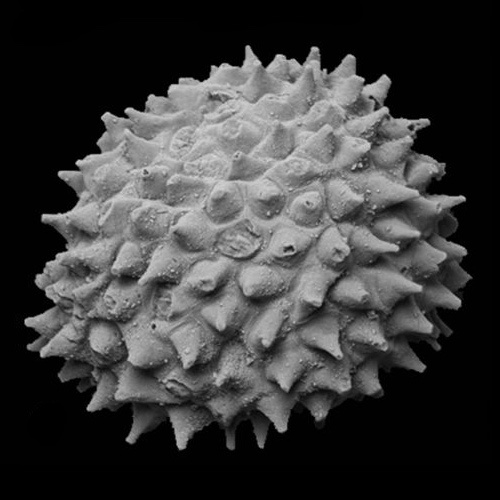
Acritarch from the Weng'an biota (570 - 609 Ma)
Wikipedia
Acritarchs: (Proterozoic to... Ordovician? Devonian? Permian? Depends on whom you ask)
- Exclusively marine
- Probably not a monophyletic group
- Organic walled cysts of... something
- Spheroids with various degrees of ornamentation
- Taxonomy is based strictly on general similarity and conveys no intentional evolutionary information.
- At least some from Doushantou have been argued to be metazoan eggs, though that obviously can't be the case for all of them.
- General similarity to resting stage of dinoflagellates (see below) sparks long-standing suspicion that they are closely related, however description of. Early Cambrian Yurtusia uniformis by Shang et al. 2020 suggests that it, at least, is a multicellular green alga.
Chitinozoa (Ordovician - Carboniferous):
- Chitinous organic-walled chambers with "neck", often arranged in chains
- Speculated by Kozlowski, 1963 to be eggs of graptolites and/or polychaet annelids. In fact, their abundance seems to track that of the former, lending strength to the argument, however no further clarification has been achieved.
Archaeplastida:
Rhodophyta: Red algae, (Cambrian (Ediacaran?) - Quaternary) including nori (M'mmm), numerous single-celled forms, and "coralline algae." Potential synapomorphies:
- Lack flagella.
- Chlorophyll a and chlorophyll b present.
- Uniquely possess phycoerythrin, a photosynthetic pigment that absorbs blue light (hence functioning at greater depths than other photosynthesizers.)
- Complex life cycle involves up to three stages of alternating generations. In the case of nori:
- Gametes fuse to form a spore bearing form
- Spores mature into a conchocelis phase that bores into the shells of marine animals.
- These release conchospores which germinate into the edible phase, which produces gametes.
(Elucidation of this revolutionized the nori industry.)
"Coraline" red algae secrete CaCO3 and are significant reef binding organisms. Indeed, up to 50% of some modern Pacific reef skeletal material is from coralline red algae.
Evolution: Red algae including the 1.2 Ga Bangiomorpha (probably) represent early multicellular eukaryotes (Butterfield, 2000.)
Rhizaria:
Radiolaria: (Cambrian - Quaternary). Heterotrophic, although some have zooxanthellae (photosynthesizing symbiots). Potential synapomorphies
- Mineralized tests - internal skeletons formed from:
- opal (hydrated silica)
- Strontium sulfate
Of these, only the first form a robust fossil record.
- Distinct morphology in which: by a perforate membrane into:
- Cytoplasm is separated into inner and outer components by the capsule, a perforated membrane
- Cytoplasm inside the capsule is termed endoplasm
- Peripheral extracapsular cytoplasm called ectoplasm [sic] characterized by gelatinous bubbles called the calymna.
- The skeleton lies within the ectoplasm.
- Thick, sticky axopodia and thin filipodia protrude from the cell for feeding.
Life cycle: Radiolarians probably have alternation of generations. Mature cells are known to break down while releasing flagellated "swarmers" that might be haploid gametes (but no one's caught them in the act of combining).
NOTE:
- Diploid = containing a full complement of chromosomes, which are grouped in homologous pairs.
- Haploid = containing half of a full complement of chromosomes, where each homologous pair is represented by one chromosome. (In animals, body cells are typically diploid and gametes (sperm and ova) are haploid.)

Chert nodule in limestone
- Major fossil forming groups are:
- Spumellaria (Silurian - Quaternary); Radially symmetrical - often roughly spherical
- Nassellaria: (Triassic - Quaternary) Radially symmetrical around long axis, forming roughly conical profile.
- Geological significance:
- As index fossils
- As source of marine silica cements, chert, etc. (right)
Foraminiferida: (Cambrian - Quaternary) In essence, "armored amoebas." Referred to as "forams." Primarily heterotrophs, feeding by means of thread-like pseudopods that project through minute openings from tests, although other feeding strategies exist including kleptoparasitism in which the chloroplasts of ingested prey are retained. Some benthic forams have zooxanthellae, including diatoms and dinoflagellates. We assume that very large extinct types, including fusulinids and nummulitids with low surface to volume ratios had primarily to depend on zooxanthellae.
- Test morphology:
- Typically with multichambered tests, although some are unilocular.
- All tests have an aperture - an opening or group of openings at the most recent chamber. Tests may, in addition, be perforate or imperforate.
- Basal forms are agglutinated, with calcareous forms becoming common in Devonian.
Life cycle involves alternation of generations:
- Diploid microspheric forms (in which the proloculus or original chamber is small) grow by serial addition of chambers or by marginal accretion.
- Asexual reproduction occurs when nucleus and cytoplasm is divided into small packages that exit the test.
- Each grows into a haploid megalosphere (i.e. big proloculus - macrosphere of some authors). Ironically, the megalosphere is typically smaller, in total than the microsphere.
- Megalospheric cytoplasm and nucleus divided into many flagellated gametes.
- Gametes fuse forming new microsphere.
- Shallow-marine benthic ancestrally. Planktonic forms appear in Jurassic, become common in Cretaceous (although suffering in K-P extinction.)
Groups of special paleontological importance:
- Fusulinida: (Late Carboniferous-Permian), up to 10 cm long
- Globogeriina: (Jurassic - Quaternary) Very common from Cretaceous onward, planktonic
- Nummulitida: (Eocene epoch of Paleogene), extremely large lentil-shaped tests.
Significance to geologists:
- As biostratigraphic index fossils (planktonic for late Mesozoic and Cenozoic, fusulinids for Late Paleozoic.)
- As reliable source of untransported carbonate shell material for stable-isotope analyses.
Alveolata: Characterized by a layer of flattened alveoli that supports the cell membrane. We focus on Dinoflagellata: (Sil. - Rec.) Dinoflagellates are typically marine, but some inhabit fresh water. Some possess chloroplasts, others don't. Chloroplasts, where present, are bound by three cell membranes rather than the original two. Perhaps they derive from eukaryotic photosynthesizing symbiotes (aka zooxanthellae). Some dinoflagellates are, themselves, zooxanthellae of multicellular metazoans. Blooms of dinoflagellates are responsible for red tides. Potential synapomorphies:
- Alveoli – cavities in cytoplasm - develop into plates forming organic walled tests
- Active stages characterized by a circumferential groove, the cingulum which houses a transverse flagellum. An additional longitudinal flagellum is at the trailing edge.
- Several different components to life cycle:
- Haploid, motile schizonts reproduce by fission.
- Schizonts act as gametes, fusing to form diploid zygotes
- Zygotes are motile for a while, but eventually encyst and enter resting phase as hypnozygotes (The wall of the hypnozygote, termed a "dinocyst" what's usually preserved in fossil record).
- Hypnozygote excysts (i.e. cytoplasm exits the dinocyst) and fissions into new schizonts. The fossil record of dinoflagellates consists mostly of hypnozgote cysts.
Note, although dinoflagellate-like fossils start in the Silurian, the good record of unambiguous dinocysts begins in the Triassic. Roughly coincides with late estimates of the end of acritarchs.
Bacillariophyceae (diatoms): (Jurassic? (definitely Cretaceous) - Quaternary) Diatoms inhabit all illuminated moist environments, including those on continents.
Morphology:
- Single chambered tests made of two silicaceous frustules. One frustrule encloses the other like the gold wrapping of Chanukkah geld or like a petrie dish and lid. They are barely motile, lacking pseudopodia or flagella.
Life cycle:
- Diploid cells reproduce asexually. With each division, each offspring receives one frustrule, and secretes another nested inside it. Thus, with each generation, final size decreases.
- Cells divide into haploid gametes.
- Gametes fuse for form a diploid auxospore, which quickly achieves maximum size before forming frustrules.
Major groups:
- Centrales: (Jurassic? - Quaternary)
primitive, probably paraphyletic, radial symmetry
- Pennales: (Eocene epoch of Paleogene - Quaternary)
derived, likely monophyletic, bilaterally symmetrical
Geological significance:
- Useful index fossils, esp. in silica rich marine environments (below CCD)
- Source of deep marine silica cement and chert
- Diatomite - sediment from diatom tests - has numerous commercial applications.
- Environmental sensitivity makes them good paleoenvironmental indicators.

Coccolithophorid
Coccolithus pelagesfrom
Wikipedia
Coccolithophyceae (coccolithophorids): (Late Triassic - Quaternary. Diversity peak in Cretaceous.) Autotrophic and exclusively marine except for one fresh-water species.
Morphology:
- Small (approx. 15-100 microns across) for eukaryotes.
- Covered with calcareous plates (coccoliths) 2 - 25 microns across, arranged into coccospheres. Small size of coccoliths limited study prior to SEM.
- Highly motile. During active stages, bear three flagella, one each at top and bottom, and a specialized haptonema at the "equator"
- Different coccolith morphs may be present in the same coccosphere. Because coccospheres invariably fall apart after death, this leads to a situation similar to that facing biostratigraphers who must distinguish sexual or life-stage morphs of whole organism body fossils.
Life cycle not well studied, but alternate between haploid and diploid phases, and can reproduce asexually through mitosis during either life stage. Different coccolith morphs may be secreted by the same species depending on life stage.
Geological significance:
- Chalk is limestone made of coccoliths. Hence "Cretaceous" - the age of chalk, from Latin "creta" - "chalk."
- Environmental sensitivity makes some taxa good paleoenvironmental indicators. Some ecological opportunist coccolithophorids mark intervals of global environmental stress.
- Despite potential caveats above, useful as index fossils.
Opisthokonta:
Fungi have a limited fossil record, although, perhaps the earliest known eukaryote fossils were of fungi (Retallack et al., 2013). Fungi apparently did have their big evolutionary moment in the Silurian and Early Devonian when giant fruiting bodies called "Prototaxites" were the tallest organism on land. (Right)
Now consider an opisthokontan group with no fossil record but which is, nevertheless important to the understanding of metazoan evolution: Choanoflagelatea, the putative sister taxon to Metazoa, characterized by a collar of cilia surrounding a single flagellum. These free-living organisms are outwardly indistinguishable from the specialized choanocytes of sponges.
- Some species of choanoflagellates secrete loricae - extracellular coverings composed of fine bars of silica.
Origins of Multicellularity
What can they tell us about the biology of creatures about to become multicellular?
There are four criteria of proper multicellularity Gould, 2011:
- Adhesion: Cells have to be able to stick together.
- Communication: Cells must be able to signal one another so the body can respond as a whole.
- Dependency: Cells must be dependent on surrounding cells for survival.
- Differentiation: Cells have to display specializations.
We've seen organisms that display some, but not all of these criteria:
- Slime molds: Temporary aggregates of amoeboid cells that display communication and (temporary) differentiation, but not adhesion or dependency.
- Colonial algae like Volvox that display adhesion but not differentiation.
 Proterospongia
Proterospongia
Proterospongia: An organism in which colonies of choanoflagellates are embedded in an extracellular jelly-like substance in which live amoeboid cells that divide and differentiate into new choanoflagellates. In this case, we see:
- Differentiation
- Dependency
- Communication
But not really adhesion. So close! Next lecture, we discuss the next big step.
Additional reading:
- Sina M. Adl, Alastair G. B. Simpson, Mark A. Farmer, et al.. 2005. The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists. Eukaryotic Microbiology. 52(5), 399–451.
- Nicholas J. Butterfield. 2000. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26(3):386-404.
- Abderrazak El Albani , Stefan Bengtson, Donald E. Canfield, Armelle Riboulleau, Claire Rollion Bard, Roberto Macchiarelli, Lauriss Ngombi Pemba, Emma Hammarlund, Alain Meunier, Idalina Moubiya Mouele, Karim Benzerara, Sylvain Bernard, Philippe Boulvais, Marc Chaussidon, Christian Cesari, Claude Fontaine, Ernest Chi-Fru, Juan Manuel Garcia Ruiz, Franćois Gauthier-Lafaye, Arnaud Mazurier, Anne Catherine Pierson-Wickmann, Olivier Rouxel, Alain Trentesaux, Marco Vecoli, Gerard J. M. Versteegh, Lee White, Martin Whitehouse, Andrey Bekker. 2014. The 2.1 Ga Old Francevillian Biota: Biogenicity, Taphonomy and Biodiversity. PlosOne, June 2014.
- S. E. Gould. 2011. Bacteria with bodies - multicellular prokaryotes. Scientific American, November, 2011.
- Patrick Keeling, Brian S. Leander, and Alastair Simpson. 2009. Eukaryota, Organisms with nucleated cells. Tree of Life Web Project, accessed 8/24/2016 at http://tolweb.org/Eukaryotes/3.
- Eunsoo Kim and Linda E. Graham. 2008. EEF2 Analysis Challenges the Monophyly of Archaeplastida and Chromalveolata. PLoS One. 2008; 3(7): e2621.
- R. Lozlowski. 1963. Sur la nature des chitinozoaires. Acta Palaeontologica Polonica. 8: 425–45
- Jeffrey D. Palmer, Douglas E. Soltis, and Mark W. Chase. 2004. The plant tree of life: an overview and some points of view. American Journal of Botany 91(10), 1437-1445.
- Gregory J. Retallack, Evelyn S. Krull, Glenn D. Thackray, Dula Parkinson. 2013. Problematic urn-shaped fossils from a Paleoproterozoic (2.2 Ga) paleosol in South Africa. Precambrian Research 235, 71-87.
- Xiaodong Shang, Pengju Liu, Malgorzata Moczydlovwska, Ben Yang. 2020. Algal affinity and possible life cycle of the early Cambrian acritarch Yurtusia uniformis from South China. Palaeontology 10.1111, 12491.
- Mark van der Giezen, Jorge Tovar. 2005. Degenerate mitochondria. EMBO Reports. 6(6), 489-589.
- Lei-ming Yin. 1997. Acanthomorphic acritarchs from Meso-Neoproterozoic shales of the Ruyang Group, Shanxi, China. Review of Palaeobotany and Palynology. 98(1-2), 15-25.
To Next Lecture.
To Previous Lecture.
To Syllabus.