The Origins and Diversity of Bilateria
Bilateria (also referred to as "Bilateralia" in older literature): Monophyletic group containing most animals. In simple terms, bilaterians are animals with a distinct front, back, top, and bottom.
Fundamental synapomorphies:
- An antero-posterior axis (rather than an oral-aboral axis)
- Bilateral symmetry (although this may be suppressed or overprinted in some)
- Presence of a flow-through gut with distinct mouth and anus.
- Triboblastic: I.e. a third germ layer, mesoderm is present in addition to the endoderm and ectoderm of cnidarians. Mesoderm replaces the passive extracellular mesoglea of cnidarians with three-dimensional living tissue.
- Body segmentation.
Bilaterian Development:

Cleavage from A. S. Romer. 1977. The Vertebrate Body.
Polarity: In all ova yolky matrial tends to concentrate at one end, yeilding:
- "Animal" pole, the non-yolky side
- "Vegetal" pole, the yolky part.
Cleavage Phase of rapid cell division with little overall growth. The zygote transforms into a hollow sphere of cells, the blastula. The space in the middle is the blastocoel
The blastula has three cell types:
- Smaller cells nearer the "animal" pole.
- Larger, yolky cells nearer the "vegetal" pole.
- A third type of cell girdling the large cells of the "vegetal" pole.

Gastrulation from A. S. Romer. 1977. The Vertebrate Body.
- Cells of the "vegetal" hemisphere form a flat plate then invaginate inward, yielding a two layered hemisphere.
- The rim of this concavity qickly closes into a small opening, the blastopore. While this is occurring, cells from the third cell type stream in through the blastopore and move forward along the inner surface. These become mesoderm. Depending on the taxon, blastopore will become either mouth or the anus.
- Endoderm: Destined to give rise to the gut tube and associated structures.
- Ectoderm: Destined to give rise to the skin and, in vertebrates, central nervous system.
- Mesoderm: Destined to give rise to a large range of internal organs (depending on the taxon.)
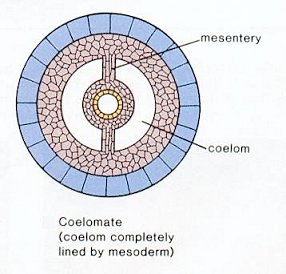
The Coelom: A characteristic feature of bilaterians is the presence of a coelom or body cavity. This feature allows for:
- increased gut expansion,
- hydrostatic structure against which muscles may act, facilitating purposeful locomotion
- use of coelomic fluid in gas and nutrient transport, breaking dependence on simple diffusion and allowing larger body size.
- Eucoelomate: In which a proper coelom develops within the mesoderm. (right)
- Pseudocoelomate: In which the coelom develops between the endoderm and mesoderm as a remnant of the blastocoel and is properly called the pseudocoel. Rotifers, kinorhnchs, nematodes, and a few other minor taxa.
- Acoelomate: In some critters, including platyhelminth flatworms, the coelom is drastically reduced or absent.
The evolution of the coelom opened many vistas for animal evolution, including significantly expanded locomotor strategies. Cnidarians show the limits of what a hydrostatic skeleton can do for an animal with a single module. Bilaterians, however, display body segmentation in which separate modules of the hydrostatic skeleton can lengthen and shorten, facilitating much more complex movement. This makes possible activities like:
- Purposeful locomotion
- Burrowing into the substrate
Specialized organs: These capabilities came at a price. Animals with only endoderm and ectoderm don't need to worry about gas exchange and elimination of nitrogenous waste, because no living cell is so far from the body surface that simple diffusion can't do the trick. Bilaterians, in contrast, usually require specialized organs for functions like:
- Gas exchange
- Nitrogenous waste elimination
- Circulation
- Coordination of the nervous system
- Sensing the environment (useful to motivate and manage locomotion)
Bilaterian Phylogeny:
First, fully appreciating the significance of segmentation as a synapomorphy of Bilateria, and the deep relationship shared by bilaterians requires an excursion into the realm of genomics:
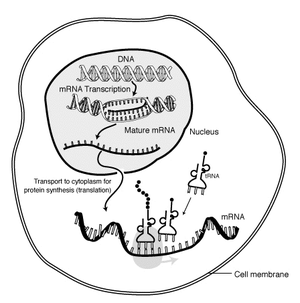
Regulatory genes: The structure of DNA was discovered in 1953, and its role as the physical repository of genes illuminated in the following decades. A brief review of how information encoded as nucleic acid is expressed as proteins goes like this:
- DNA at rest in the nucleus is a double helix (spiral staircase) whose corresponding base pairs are connected through weak bonds.
- RNA polymerase separates the DNA helices and uses one of them as a template for assembling a single-strand messenger RNA molecule (mRNA).
- mRNA passes from the nucleus into the cytoplasm, where it encounters the two components of the ribosome.
- The ribosome grabs passing transfer RNA (tRNA) molecules. tRNA have nucleotide triplets (codons) exposed at one end. Each nucleotide triplet combination is associated with a specific amino acid, that is bound to the opposite end of the tRNA.
- The ribosome matches nucleotide triples on the mRNA to corresponding tRNAs, moving down the mRNA strand. Amino acids connected to adjacent tRNAs bind to form a protein, an amino acid polymer, that forms part of the cell's structure or does work in it.
Link to simple animation or to a more detailed explication.
The magic of the arrangement is that DNA is only transcribed into mRNA when the protein it codes for is needed. E.G.: The bacterium Escherichia coli freely metabolizes glucose, but if glucose is lacking, and lactose is present, it can metabolize it by producing an enzyme, beta-galactosidase, that breaks lactose down in to glucose and galactose. But how does it know when to make beta-galactosidase?
The work of François Jacob and Jacques Monod established the answer (Nobel Prize in 1965): Adjacent to the gene for beta-galactosidase (and other proteins involved in lactose metabolism) is a small controlling region gene to which a protein, the lac repressor binds like a padlock. This blocks RNA polymerase from unzipping the DNA and making mRNA. But, lactose, itself, binds to the lac repressor causing it to fall off of the DNA strand and allowing RNA polymerase to do its thing. Once the lactose has been metabolized, the lac repressor reattaches and transcription ceases. Animation.
Of course, the lac repressor protein is coded for, in turn, by a repressor gene whose activity may be regulated by other repressors. Indeed, the expression of genes as proteins is the result of complex cascading interactions of regulatory and structural genes and their protein products. Why are we even discussing this?
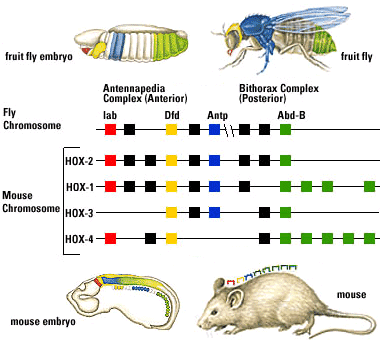
Fruit flies are a favorite model for geneticists, with short generation spans and interesting mutations that often effect entire sections of the body (modifying or eliminating body segments and/or the appendages that grow from them). Investigation into these segmentation-altering mutations revealed that they can be caused by mutations to eight genes. What makes this interesting:
- The genes are close to one another on a single chromosome, and are physically lined up in the same order as the segments of the body they effect.
- Each gene (of ~ 1000 base pairs) contains a nearly identical sequence of about 60 base-pairs called homeoboxes.
But it gets better: The search for homologs to fruit fly Hox genes found them in almost every animal surveyed. Mammals, for example, have four clusters of Hox gene homologs, in each of which the genes occur in the same order on the chromosome as the regions for which they code.
This is huge.
The fruit flies and mammals belong to the two major clades of bilaterians, and their last common ancestor lived during (or before) the Ediacaran, and yet they share important regions of the genome.
The Bilaterian cladogram:
Working out the bilaterian pattern in detail is a difficult problem that is slowly being illuminated by molecular methods. In its broad strokes, however, there is a clear pattern of two large strongly supported clades:- Deuterostomia: Includes chordates and echinoderms plus some minor groups. Synapomorphies include:
- A distinct deuterostomous pattern of development (right - b) where the blastopore becomes the anus and the mouth develops from a secondary opening.
- Development of the coelom is enterocoelomate, in which the coelom originates as an out-pouching of the gut tube.
- Development is indeterminate development: i.e. the fate of a given cell is fluid and based on inductive relationships with neighboring cells and tissues.
- Cleavage of embryonic cells is radial , giving embryos the ability to twin.
- Nerve chord forms dorsal to gut tube in adults.
- Protostomia: Includes arthropods, mollusks, annelid worms, brachiopods, and bryozoans plus some minor groups. Synapomorphies include:
- Blastopore gives rise directly to mouth and anus by elongating and "pinching off." (right - a)
- Development of the coelom is schizocoelomate, in which the coelom forms from direct cavitation of the mesoderm.
- Development is determinate, in which cell fates are absolute.
- Cleavage of embryonic cells is spiral, or arguably derived from a spiral pattern.
- Nerve chord ventral to gut tube in adults.
Within Protostomia are two well-supported clades:
- Ecdysozoa: Includes arthropods, nematodes, onychophorans, and other critters that regularly shed an external cuticle.
- Spiralia: ("Lophotrochozoa" of many authors. Prothero uses "Spiralia" alleged to be the senior synonym.) Includes mollusks, annelids, brachiopods, phoronids, and bryozoans. United by molecular characters.
Further divided into:
- Trochozoa:
- Synapomorphy: Trochophore larva
- Annelida and Mollusca, the only members with substantial fossil records.
- "Lophophorata"
- Formerly thought to be monophyletic on the basis of morphology, especially possession of a lophophore, a ciliated suspension-feeding organ, but more recently argued to be either a polyphyletic group of lophophore-bearing lophotrochozoans (Passamaneck and Halanych, 2006), paraphyletic (Helmkampf et al., 2007), or genuinely monophyletic (Jang and Hwang, 2009).
- Trochozoa:
Within Deuterostomia are also two well-supported clades:
- Ambulacraria: Includes echinoderms and hemicordates.
- Chordata: Includes vertebrates, tunicates, and some minor groups.
As promised: Bilaterian Origins:
CURRENTLY UNKNOWN.
What we do definitely know:
- The Ediacaran Kimberella quadrata (right) has been argued to be a stem mollusk (Fedonkin et al., 2007), and certainly looks like one. If so, the radiation of the major bilaterian groups had definitely happened by 555 ma.
- The Ediacaran Cloudina specimen shown in the previous lecture shows a surprise. The yellow arrow indicates a place where it has been bored into by a minute predator! Among living creatures, only bilaterian grade organisms are capable of this.
- Some Ediacaran trace fossils seem to have been made by organisms capable of shallow burrowing and with front and back ends. Presumably bilaterian. Claims of trace fossils as old as 2.1 ga have been made. Currently the oldest that meets clear criteria for bilaterian-style motion are described by Pecoits et al. 2012 (right) and dated at least 585 ma.
The identity of these and later traces remains unknown, and yet, if we have Ediacaran worm-traces and a stem mollusk, early bilaterians must be out there. Why are we missing them?
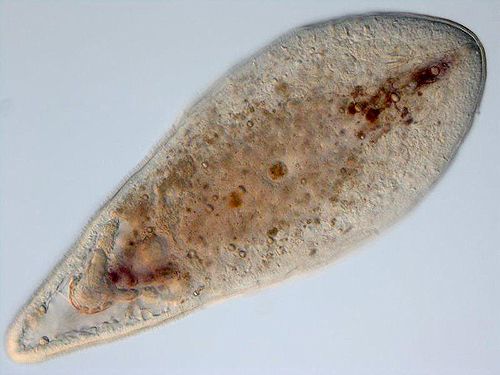
Too small to identify: The notion that bilaterians evolved through paedomorphosis from an ancestor resembling a cnidarian planula larva has had support since the early 20th century. Indeed, acoelomorph flatworms resemble what one might expect of such a creature.
Note: "flatworms" making up the traditional phylum Platyhelminthes are Polyphyletic. The "platyhelminthine" subgroup Acoelomorpha, however, appears monophyletic and may have a basal position on the bilaterian tree (Ruiz-Trillo and Paps, 2016.) Acoelomorphs are tiny (2-4 mm.) and display features that seem transitional between non-bilaterian and bilaterian grades, and have been suggested as models for the ancestral bilaterian (Hejnol and Martindale, 2007). They possess:
- Mesoderm
- An anteroposterior axis
- A concentration of sensory neurons near their front end
- Gastrulation that begins at the vegetal pole (in cnidarians and ctenophores, it happens at the animal pole.)
- Five Hox genes
- An anus - their guts are blind-ended
- A coelom
- A proper central nervous system
- Segmentation
Note: Today one frequently hears of the identification of genes associated with the formation of eyes (PAX6), central nervous systems, etc. being widespread among Bilateria, the implication being that the last common ancestor of Bilateria must possess the features involved. Careful! The genes may be homologous, but we can't know how they were expressed in the context of a simpler organism. Holland, 2003, for example, found that genes associated with the central nervous systems of protostomes and deuterostomes that had them were expressed in a decentralized epidermal nerve network of a hemichordate - an animal lacking a brain.
Possible body fossils:
- Chen et al. 2000 and Hagdorn et al. 2006 describe a suite of phosphatized embryos including those of cnidarians and putative bilaterians from the Ediacaran Doushantuo Fm. (590-550 ma). For unknown reasons, bilaterians are preserved in the gastrula stage. This interpretation has been questioned sbsequently, with critics observing that the bilaterian embryos could as easily represent giant sulfur-reducing bacteria (Bailey et al., 2006) or resting stages of single celled eukaryotes (Huldtgren et al., 2011).
- Chen et al., 2004 describe minute (0.5 mm) fossils of alleged adult bilaterian Vernanimalcula guizhouena (right), complete with anterior sensory organs, gut tube, and coelom. This, too, has been harshly critiqued and reinterpreted as a diagenetically altered encysted single-celled eukaryote. But then, it does resemble what we expect to see in an early bilaterian. Decide for yourself if this strengthens the identification or renders it suspect!

Sea pen Ptilosarcus gurneyi
Is it possible that we are seeing bilaterian ancestors and not recognizing them? Dewell 2000 suggests that bilaterians derive from modular colonial forms. In living cnidarians, such as sea pens (right) we often see modular colonies of zooids assume coherent and predictable shapes. In siphonophores, we even see zooids achieve sophisticated division of labor. Dewell asks if the bilaterian body could represent a particularly well-integrated colonial form. It would resolve the enigma of the absent bilaterians by suggesting that the ancestors of proper bilaterians might lie among the diversity of known modular Ediacaran weirdos.
One complication:
Remember Hox genes? According to Ryan
This issue awaits the discovery of key fossils. Stay tuned.
- Jake V. Bailey, Samantha B. Joye, Karen M. Kalanetra, Beverly E. Flood, and Frank A. Corsetti. 2006. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature 445, 198-201.
- Jun-Yuan Chen, David J. Bottjer, Paola Oliveri, Stephen Q. Dornbos, Feng Gao, Seth Ruffins, Huimei Chi, Chia-Wei Li, Eric H. Davidson. 2011. Small Bilaterian Fossils from 40 to 55 Million Years Before the Cambrian. Science 305(5681): 218-222.
- Jun-Yuan Chen, Paola Oliveri, Chia-Wei Li, Gui-Qing Zhou, Feng Gao, James W. Hagadorn, Kevin J. Peterson, and Eric H. Davidson. 2000. Precambrian animal diversity: Putative phosphatized embryos from the Doushantuo Formation of China. Proceedings of the National Academy of Sciences 97(9): 4457-4462.
- Ruth Ann Dewel. 2000. Colonial origin for Eumetazoa: major morphological transitions and the origin of bilaterian complexity. Journal of Morphology 243(1): 35–74.
- M. A. Fedonkin, A. Simonetta, and A. Y. Ivantsov. 2007. New data on Kimberella, the Vendian mollusc-like organism (White Sea region, Russia): palaeoecological and evolutionary implications. In in Vickers-Rich, Patricia; Komarower, Patricia, Eds. The Rise and Fall of the Ediacaran Biota, Special publications, 286, London: Geological Society, pp. 157–179
- James W. Hagadorn, Shuhai Xiao, Philip C. J. Donoghue, Stefan Bengtson, Neil J. Gostling, Maria Pawlowska, Elizabeth C. Raff, Rudolf A. Raff, F. Rudolf Turner, Yin Chongyu, Chuanming Zhou, Xunlai Yuan, Matthew B. McFeely, Marco Stampanoni, Kenneth H. Nealson. 2006. Cellular and Subcellular Structure of Neoproterozoic Animal Embryos Science 314(5797): 291-294.
- Martin Helmkampf, Iris Bruchhaus, Bernhard Hausdorf. 2008. Multigene analysis of lophophorate and chaetognath phylogenetic relationships. Molecular Phylogenetics and Evolution 46(1): 206-214.
- Andreas Hejnol, Mark Q Martindale. 2007. Acoel development supports a simple planula-like urbilaterian. Philosophical Transactions of the Royal Society 363(1496).
- Nicholas D. Holland. 2003. Early central nervous system evolution: an era of skin brains? Nature Reviews Neuroscience 4, 617-627.
- Therese Huldtgren, John A. Cunningham, Chongyu Yin, Marco Stampanoni, Federica Marone, Philip C. J. Donoghue, Stefan Bengtson. 2011. Fossilized Nuclei and Germination Structures Identify Ediacaran “Animal Embryos” as Encysting Protists Science 334(6063): 1696-1699.
- Kuem Hee Jang and Ui Wook Hwang. 2009. Complete mitochondrial genome of Bugula neritina (Bryozoa, Gymnolaemata, Cheilostomata): phylogenetic position of Bryozoa and phylogeny of lophophorates within the Lophotrochozoa. BMC Genomics200910:167.
- Yale Passamanecka and Kenneth M. Halanych. 2006. Lophotrochozoan phylogeny assessed with LSU and SSU data: Evidence of lophophorate polyphyly. Molecular Phylogenetics and Evolution 40(1): 20-28.
- Ernesto Pecoits, Kurt O. Konhauser, Natalie R. Aubet, Larry M. Heaman, Gerardo Veroslavsky, Richard A. Stern, Murray K. Gingras. 2012. Bilaterian Burrows and Grazing Behavior at >585 Million Years Ago. Science 336(6089): 1693-1696.
- Iñaki Ruiz-Trillo and Jordi Paps. 2016. Acoelomorpha: earliest branching bilaterians or deuterostomes?. Organisms Diversity & Evolution 16(2): 391–399.
- Joseph F. Ryan, Maureen E. Mazza, Kevin Pang, David Q. Matus, Andreas D. Baxevanis, Mark Q. Martindale, John R. Finnerty. 2007. Pre-Bilaterian Origins of the Hox Cluster and the Hox Code: Evidence from the Sea Anemone, Nematostella vectensis. Plos|One January 24, 2007.
To Previous Lecture.
To Syllabus.